valence electrons for as|valence electrons quizlet : Manila There are two ways to find the number of valence electrons in Arsenic (As). The first is to use the Periodic Table to figure out how many electrons Arsenic h. ROBUST AND VERSATILE: Wherever hydraulic tasks need to be performed, HYDAC hydraulic accumulators can help. They are versatile, make your machine more convenient to use, secure your hydraulic system and are used to increase the energy efficiency of hydraulic systems and for many other tasks.Do you want to watch Pure Taboo - Why Deprive Yourself with Nicole Kitt? Full porn video is available for you Online. 🎀 HAPPY HOLIDAYS - ENJOY BRAZZERS FREE FOR 7 DAYS!!!
valence electrons for as,There are two ways to find the number of valence electrons in Arsenic (As). The first is to use the Periodic Table to figure out how many electrons Arsenic h.
valence electrons for as valence electrons quizlet Mar 23, 2023 Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2s subshell and .

You may assume the valences of the chemical elements—the number of .Learn how to determine the number of valence electrons for an element using the periodic table. An atom's valence electrons are the electrons in its outermost shell. In the . Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical .
valence electrons quizletIn chemistry and physics, valence electrons are electrons in the outermost shell of an atom, and that can participate in the formation of a chemical bond if the outermost shell .Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the 1s 1 s sublevel are called . Valence electrons are outer shell electrons for main group elements. For the transition metals with partially-filed d shells, valence electrons are those electrons outside the noble gas core. The number .valence electrons for as Valence electrons: For main group elements (i.e s-block and p-block elements), the valence electrons are the electrons present in the outermost orbit. But for most of the transition and inner transition .Valence electrons are responsible for the reactivity of an element. They determine how "willing" the elements are to bond with each other to form new compounds. If the valence shell of an element is full, such as with a noble gas, then the element does not want to gain or lose an electron. For example, alkali metals, which all have a valency of . H+ is just a proton, so no electrons would be present. In covalent bonding, the electrons that form the covalent bond do not have to come from each atom. There are instances when an atom . Solution. Steps for Writing Lewis Structures. Example 15.4.1 15.4. 1. 1. Determine the total number of valence electrons in the molecule or ion. Each H atom (group 1) has 1 valence electron, and the O atom (group 16) has 6 valence electrons, for a total of 8 valence electrons. 2.
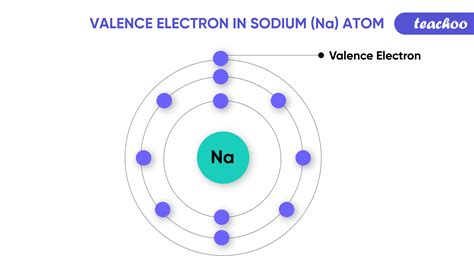
An element’s valence was historically determined by how many hydrogen atoms it could bond to (which is determined by how many valence electrons it has available for bonding): for example, carbon can form CH 4 so it has a valence of 4, and 4 valence electrons. On the other hand, nitrogen can form NH 3 so it has a valence of 3, and 3 valence . Valence electrons are those electrons that reside in the outermost shell surrounding an atomic nucleus. Valence electrons are of crucial importance because they lend deep insight into an element’s chemical properties: whether it is electronegative or electropositive in nature, or they indicate the bond order of a chemical compound – the . The layers of electrons around an atom don't always follow a straightforward pattern, so you can't always predict an element's behavior just by counting these outer electrons. Nevertheless, it's useful to know the most common valences. Here is a table of element valences. Arsenic ion (As 3-) electron configuration. The ground state electron configuration of arsenic is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 3. This electron configuration shows that the last shell of arsenic has five electrons. Therefore, the valence electrons of arsenic are five.
valence electrons for as|valence electrons quizlet
PH0 · valence electrons worksheet
PH1 · valence electrons quizlet
PH2 · valence electrons for kids
PH3 · valence electrons calculator
PH4 · list of valence electrons for each element
PH5 · how do you find valence electrons
PH6 · arsenic valence electron configuration
PH7 · arsenic valence electron
PH8 · Iba pa